Chem 1200
Reactions XII
The electrode at the anode is the first one to be listed in the notation.
The electrode at the cathode is the last one to be listed in the notation. Both electrodes are connected through a wire. The electrodes need not be participating in the reaction, (In this example, Zn is a reactant) an electrode can be an inert metal whose sole purpose is to be connected to a wire.
Let us go back to the example of water flowing from one tank to another. In this case, we will consider more than two tanks.
Like height, which is independent of the size of the water tanks, the cell potential is also independent of the amount of reactants. It is an intensive quantity. This always happens when the quantity is obtained by dividing an extensive quantity by another extensive quantity. An example is density which is mass divided by volume. Both mass and volume are extensive for these depend on how much material one has, but density is intensive. The potential is defined as the energy that can be derived from a redox reaction divided by the amount of electrons (or charge) that passes through the wire. Energy is in Joules and the Coulomb is a unit of charge. The unit of potential is volts.
Analogous to the height of tanks, which enables us to predict the direction of flow, we have the electromotive force (emf) or potential to help us decide what is going to get reduced and what is going to get oxidized.
Like the tanks where we measure a difference in height, in electrochemistry, we will use a difference in potential.
By comparing various cells against the standard hydrogen electrode, one can obtain cell potentials for other half-reactions. These are tabulated in standard reduction potential tables, such as the one shown below.
These are standard reduction potentials and in the above table, the half-reactions are listed according to the magnitude of the potential, listing the ones with high positive values first. We can then tell from this list that the species listed first are reduced more spontaneously. These species are therefore strong oxidizing agents. The ones near the bottom are the not likely to occur so the reverse reaction is more favored. Therefore, Li(s), for example, is very reactive. Li(s) can get easily oxidized, so Li(s) is a good reducing agent. Recalling the periodic table, we see that a halogen like fluorine is a good oxidizing agent, agreeing with the fact that fluorine is a very electronegative element. On the other hand, lithium, an alkali metal, is electropositive, so it behaves as a reducing agent, lithium metal is eager to give electrons.
The following is an example of how a table of standard reduction potentials is used.
Before we proceed, we need to review thermodynamics and equilibrium:
In general:
The Nernst equation gives us the potential under nonstandard conditions.
We do this when Q is not equal to 1, that is, not all concentrations are equal to 1M, (and/or) not all partial pressures equal 1 atm.
We are more familiar with base 10, so another form of the Nernst equation is seen below.
We can simplify the Nernst equation further at T = 298 K:
Before we proceed, we need to review thermodynamics and equilibrium:
In general:
The Nernst equation gives us the potential under nonstandard conditions.
We do this when Q is not equal to 1, that is, not all concentrations are equal to 1M, (and/or) not all partial pressures equal 1 atm.
We are more familiar with base 10, so another form of the Nernst equation is seen below.
We can simplify the Nernst equation further at T = 298 K:
The value of n (the number of electrons) depends on how the reaction is written. If we had written the reaction in the following manner instead:
2 Zn(s) + 4H+(aq) à 2 Zn2+(aq) + 2 H2(g)
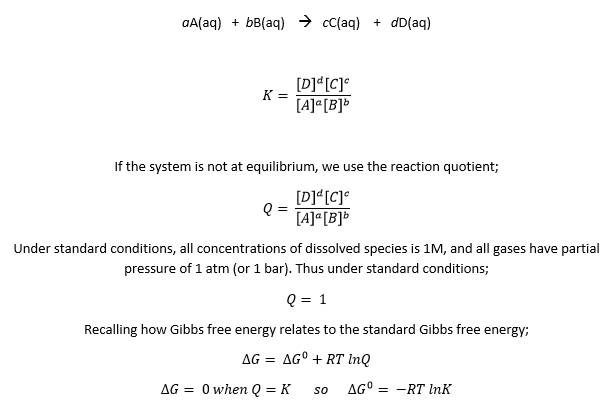
The value of n is 4. And the reaction quotient is also different:
This new Q is simply the square of the previous Q. Since Q is inside the logarithm function, when Q is squared the logarithm is doubled. This, however, is canceled by the fact that n is also doubled. Thus, with the Nernst equation, we can clearly see the potential as an intensive quantity. It does not depend on how we write the balanced equation.
When we double the reaction, Q gets squared, log Q is therefore doubled, but n is doubled as well when we double the reaction. Thus, the doubling gets canceled.
The Nernst equation tells us that the actual cell potential depends on the concentrations of dissolved species and the partial pressures of gases that are involved in the reduction-oxidation reaction. In this specific case, we see that the potential is influenced by the concentration of Zn(II), H+, and the partial pressure of H2. Therefore, if both [Zn(II)] and PH2 are kept constant, we find the cell potential as a function of pH.
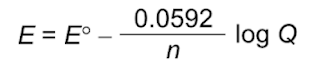
No comments:
Post a Comment