Chem 1200
Reactions XII
Before we proceed, we need to review thermodynamics and equilibrium:
In general:
The Nernst equation gives us the potential under nonstandard conditions.
We do this when Q is not equal to 1, that is, not all concentrations are equal to 1M, (and/or) not all partial pressures equal 1 atm.
We are more familiar with base 10, so another form of the Nernst equation is seen below.
We can simplify the Nernst equation further at T = 298 K:
Before we proceed, we need to review thermodynamics and equilibrium:
In general:
The Nernst equation gives us the potential under nonstandard conditions.
We do this when Q is not equal to 1, that is, not all concentrations are equal to 1M, (and/or) not all partial pressures equal 1 atm.
We are more familiar with base 10, so another form of the Nernst equation is seen below.
We can simplify the Nernst equation further at T = 298 K:
The value of n (the number of electrons) depends on how the reaction is written. If we had written the reaction in the following manner instead:
2 Zn(s) + 4H+(aq) à 2 Zn2+(aq) + 2 H2(g)
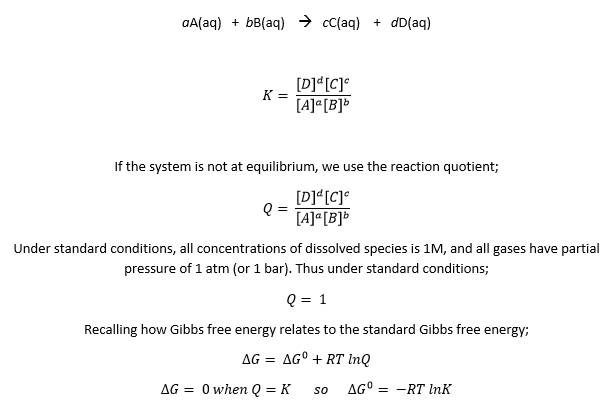
The value of n is 4. And the reaction quotient is also different:
This new Q is simply the square of the previous Q. Since Q is inside the logarithm function, when Q is squared the logarithm is doubled. This, however, is canceled by the fact that n is also doubled. Thus, with the Nernst equation, we can clearly see the potential as an intensive quantity. It does not depend on how we write the balanced equation.
When we double the reaction, Q gets squared, log Q is therefore doubled, but n is doubled as well when we double the reaction. Thus, the doubling gets canceled.
The Nernst equation tells us that the actual cell potential depends on the concentrations of dissolved species and the partial pressures of gases that are involved in the reduction-oxidation reaction. In this specific case, we see that the potential is influenced by the concentration of Zn(II), H+, and the partial pressure of H2. Therefore, if both [Zn(II)] and PH2 are kept constant, we find the cell potential as a function of pH.
The reason why we see a potential because of a difference in pH between two compartments is again the dependence of the potential on the concentration of species.
Since the cell potential is related to Gibbs free energy, we can then derive from measured cell potentials, the equilibrium constant for the reaction. Here we note again that a positive potential corresponds to a spontaneous reaction, and provides a large equilibrium constant indicating that products are favored.
At equilibrium, the change in the concentration of reactants and products is now zero. No noticeable reaction is occurring. The cell potential at equilibrium is zero and the reaction quotient, Q, is now equal to the equilibrium constant, K. We can then find the relationship between the standard cell potential and the equilibrium constant:
The dependence of the potential on concentration is highlighted in a special type of electrochemical cell called the concentration cell. The following is an example.
The equation above shows that the cell potential is present when the concentration of Cr(III) is different between the anode and cathode compartments. More importantly, the potential of a concentration cell is positive only if the concentration of Cr(III) is higher at the cathode than it is in anode. This makes sense since Cr(III) is a reactant at the cathode, and is a product at the anode. With Le Chatelier's principle, it is important that we have more of the reactant for a reaction to move forward, thus, Cr(III) should be at a higher concentration at the cathode where Cr(III) is the starting material. We expect the opposite if an anion is involved. The anion, for instance, a chloride ion, is oxidized at the anode into chlorine gas, while chlorine gas is reduced to chloride at the cathode. In this case, the chloride ion is the reactant at the anode. Thus, for the cell potential to be positive, the anion should be at a higher concentration at the anode. This should be easy to remember, a cation should be higher in concentration at the cathode while an anion should be at a higher concentration at the anode, for the cell potential to be positive.
In biology, concentration differences across cell membranes is crucial in establishing cell potentials. This is illustrated in the following.
Now, we will go through a series of useful galvanic cells.
In the cell notation below, it is clear that a "/" is not exclusively used to denote a change in phase, it is also used between solids where the oxidation number changes. Pb is zero when Pb is metal, and Pb is +2 in PbSO4. At the anode, Pb is oxidized. We see a similar use of "/" at the cathode. Here, PbO2 is reduced to PbSO4, and Pb metal is simply acting as an electrode for connection to a wire. These compounds are all in the solid phase, but these are not in the same solid phase (These are not alloys.), thus, a "/' is placed between these solids. The // denoting a salt bridge is not present because the salt bridge is actually specified /H2SO4(aq)/. Sulfuric acid is enclosed by "/".
When the reaction inside an electrochemical cell is not spontaneous, we now have what we call an electrolytic cell. This is the opposite of a galvanic cell. It cannot operate on its own. It needs an external power source to drive the reaction. In the following example, at the cathode, sodium metal (an alkali metal - which is very electropositive) is produced from the sodium cation. What naturally occurs is the opposite. Sodium, being electropositive, loses an electron, to form the sodium ion, which has an octet configuration. What the cell is trying to achieve therefore is opposite to what happens naturally or spontaneously. On the other hand, at the anode of the following cell, chloride ions are being reduced to chlorine gas. Again, this is opposite to what halogens do. Chlorine needs an extra electron to achieve an octet configuration. Yet, in the cell below, we are trying to oxidize chloride ions back into chlorine gas.
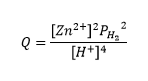


No comments:
Post a Comment