Chem 1200
Kinetics I
Before we begin the new topic, let us consider the lithium battery. It is one example in which both electrodes (Cu and Al) do not participate in the reaction.
And here is one more problem in electrochemistry.
A balanced chemical equation tells us only the stoichiometry, the nature of the reactants and the products. It does not provide us information regarding how the reaction really occurs.
The process, the step-by-step account by which a given reaction occurs is called the reaction mechanism. Why is it important that we understand the mechanism behind a reaction? First, a more detailed knowledge of how things occur never fails to interest a chemist. Second, with an understanding of how a reaction occurs, adjustments can be made so as to maximize production of a desired compound. Third, knowing the details of how a reaction occurs, we can better anticipate events that are about to happen.
As an example, we have nucleophilic substitution for organic compounds, a very important set of reactions for organic chemistry. The general scheme for this reaction is illustrated in the following:
(Route 1) [SN1] First-order nucleophilic substitution (two steps):
Rate = kSN1 [H3CX]
H3CX --> H3C+ + X- (slow)
H3C+ + Y- --> H3CY
(Route 2) [SN2] Second-order nucleophilic substitution
Rate = kSN2 [H3CX] [Y-]




On the other hand, the SN2 mechanism yields exclusively the product resulting from a back-side attack of Y:

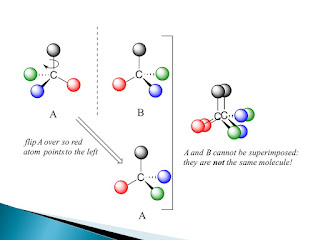
When the four atoms attached to C are different, their three-dimensional arrangement around the C atom is important. Recall that the geometry around C with 4 single bonds is approximately tetrahedral:
And there are plenty of compounds that have a C attached to four different groups. For instance, all amino acids (except for glycine) contain such a C.

This is just one example why we, as chemists, need to know the details of how a chemical reaction occurs (kinetics).
A chemist may imagine how molecules collide and rearrange to form products. A chemist may dream of elaborate or simple reaction mechanisms, but the fruitfulness of such mechanism lies in how it compares with what we observe. The rates of reaction can be affected by several factors:
The concentrations of the reactants - Most reactions proceed faster when the concentration of the reactants is increased. The dependence of the rate on concentration is summarized in a rate law.All of the above are macroscopic evidences of factors that affect how fast a reaction proceeds. The role of the chemist is to extract these dependencies quantitatively and offer a molecular explanation. And the explanation is only as good as it matches all the observed data.
The temperature at which the reaction occurs - Most reactions also proceed faster when the temperature is raised.
The presence of a catalyst - Most biochemical reactions will not occur without the aid of proteins which act as enzymes.
The surface area of solid or liquid reactants or catalysts - Reactions that involve solids (for example, when we boil potatoes, cutting them into smaller pieces hasten their cooking) often occur faster when the exposed surface area of the solid is increased.
Recall what we learned in chemical equilibrium:
The subject of kinetics is normally divorced from chemical equilibrium. This is true especially when one is considering energies. As you have seen before, the difference in energies of the reactants and products is independent of the reaction mechanism. In addition, you will see later that although catalysts affect rates of reactions, they do not change chemical equilibrium. Thus, it is appropriate to separate kinetics from thermodynamics. However, completely divorcing kinetics from thermodynamics is not entirely correct. Principles governing chemical equilibrium are related in one way or another to dynamics. One should not forget that a simple dynamic equilibrium consists of two opposing reactions occurring at the same rate. Kinetics is therefore central to an understanding of chemical equilibrium.
Chemical rates are changes in concentration over a time interval.
Rates can be either an (1) average or (2) instantaneous.
The advantage of an (1) average rate is they are easy to calculate. The disadvantage is they tend to be very general and not specific at a specific time when the rate is not constant.
The advantage of an (2) instantaneous rate is it gives very specific exact information. The disadvantage is the time it takes to set up and make the calculation.
Average rates:
change in molar concentration of reactants, R: Δ[R] = [R]t2 - [R]t1
divided by the time interval Δt = t2 - t1
Average rate of consumption of R = - "Δ[R]" /"Δt"
Note on the “-” meaning reactants disappear.
For products P = "Δ[P]" /"Δt"
Remember that [ ] stands for concentration or mol·L-1.
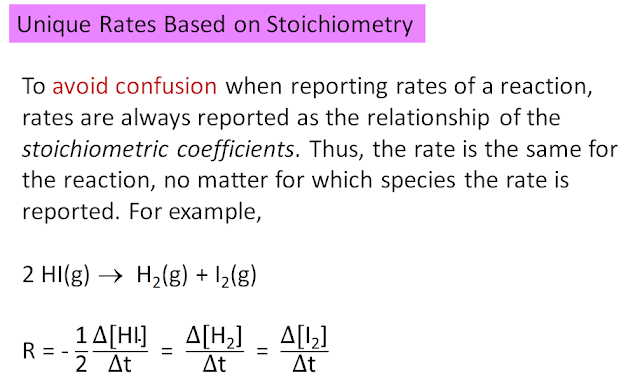
Please take note that the sign for the reaction rate corresponds to how the reaction is written. Rates are always expressed as either a rate of the disappearance of a reactant or a rate of appearance of a product. Thus, it matters how the reaction is written. In the above example, the reaction is written in reverse compared to what was presented previously.
Reaction rates: A balanced chemical equation tells us only the stoichiometry, the nature of the reactants and the products. Although a balanced chemical reaction does not normally give a more detailed description of how a reaction occurs, it indicates that the rate of disappearance of a reactant should be directly related to the rate of appearance of a product. In general:
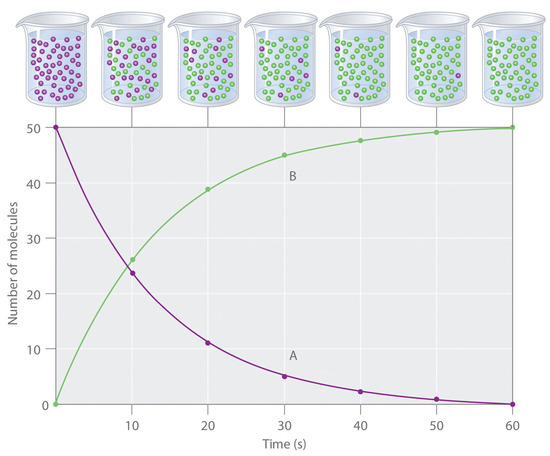
For example in a reaction A --> B, one may be able to monitor either the amount of A or the amount of B present as a function of time:


No comments:
Post a Comment