Chem 1200
Kinetics II
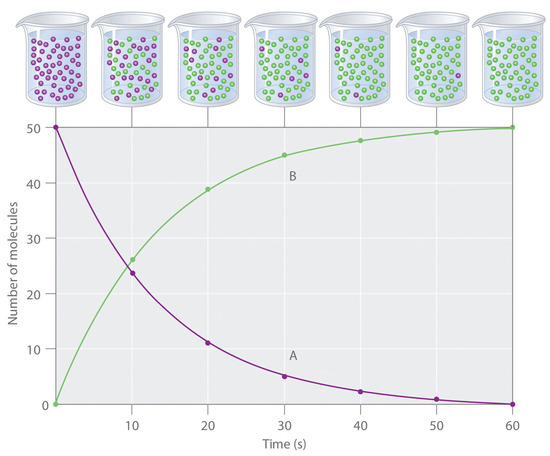
For example in a reaction A --> B, one may be able to monitor either the amount of A or the amount of B present as a function of time:
These two curves are related to each other because of stoichiometry. Remember, by stoichiometry the rate at which the concentration of a product changes is directly related to the rate at which the concentration of the reactant changes. It is possible to convert the amount of A left to the the amount of B formed, and vice-versa.
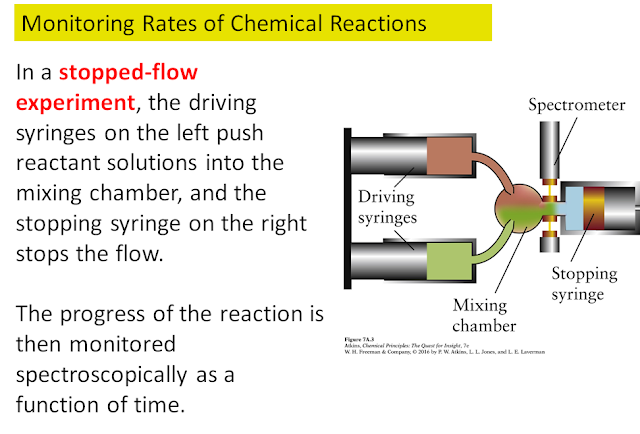
Below is an example of how the rate of a reaction can be measured:
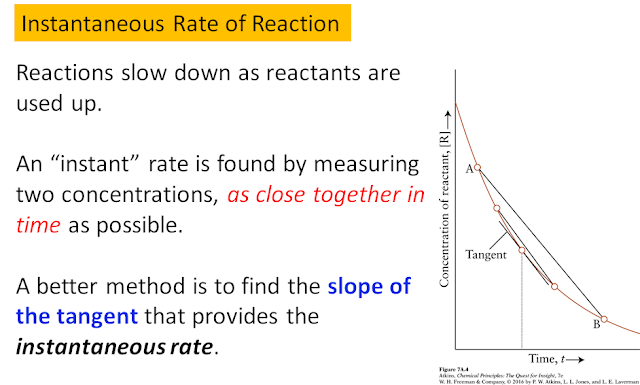
As mentioned before, the rate of a reaction can be influenced by the concentration of the reactants. Hence, rarely do we find the concentration or amount of reactant changing linearly with time. As the reaction progresses, the reactant gets consumed, therefore its concentration or amount is changing with time. This can induce a change in the rate of the reaction. Oftentimes, we see the reaction slowing down as time progresses. Thus, it is useful to describe an instantaneous rate of reaction (one that is specified at a given point in time). For this reason, rates are usually defined with differentials which signify infinitesimal (very small) changes. The graphical description of an instantaneous rate is given below:
Below is an example of how the rate of a reaction can be measured:
As mentioned before, the rate of a reaction can be influenced by the concentration of the reactants. Hence, rarely do we find the concentration or amount of reactant changing linearly with time. As the reaction progresses, the reactant gets consumed, therefore its concentration or amount is changing with time. This can induce a change in the rate of the reaction. Oftentimes, we see the reaction slowing down as time progresses. Thus, it is useful to describe an instantaneous rate of reaction (one that is specified at a given point in time). For this reason, rates are usually defined with differentials which signify infinitesimal (very small) changes. The graphical description of an instantaneous rate is given below:
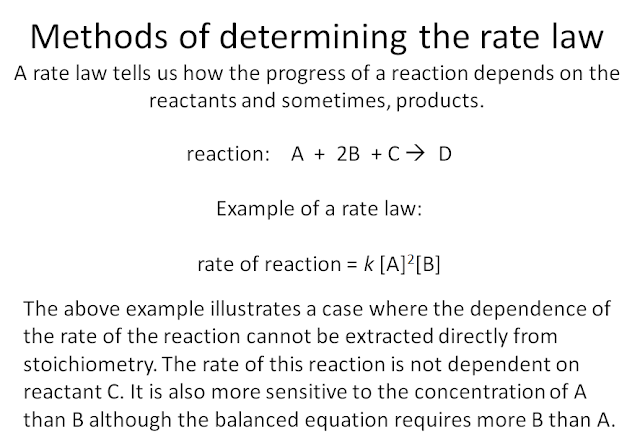
In kinetics, we must first measure the rate of a reaction. And we accomplish this by monitoring the change in concentration of the reactants, how fast are these disappearing, or by measuring the change in concentration of the products, how fast are these appearing. Only after a measurement can we then examine factors that can possibly influence how fast a reaction occurs. Since reactions require an encounter between molecules, it is reasonable to suspect that the rate of a reaction depends on the concentration of the reactants. As the concentration rises so does the chance of reactant molecules or ions colliding against each other. So we need to observe the rate of a reaction at different concentrations. How a rate of a reaction experimentally depends on concentration is summarized in what we call a rate law.
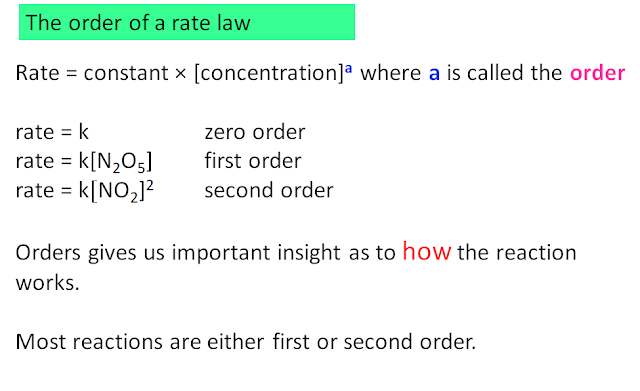
This reaction is third-order overall, second-order in A, first-order in B, and zero-order in C.
Methods of Initial rates - Measuring the instantaneous rate of a reaction at time=0 several times with various starting concentrations of the reactants. Here is an example: Rate Data for the reaction of Ammonium and Nitrite Ions in Water at 298 K.
Experiment
numberInitial NH4+
concentration, MInitial NO2-
concentration, MObserved Initial
rate, 10-7 M/s1 0.0100 0.200 5.4 2 0.0200 0.200 10.8 3 0.0400 0.200 21.5 4 0.0600 0.200 32.3 5 0.200 0.0202 10.8 6 0.200 0.0404 21.6 7 0.200 0.0606 32.4 8 0.200 0.0808 43.3
By comparing two experiments (for example 1 and 2) in which only one of the reactant's concentration is varied, in this particular case, the nitrite concentration is maintained while the ammonium concentration is doubled. The result is the rate for experiment 2 is twice as fast as that of experiment 1, indicating that this reaction depends linearly on the concentration of ammonium (first order with respect to ammonium).
rate = k[A]x[B]y then the reaction is x order with respect to [A], y order with respect to [B], and overall x+y order. The proportionality constant k is called the rate constant. As we will see later, the temperature dependence of the rate of a reaction manifests in k. In this course, we will stay within simple rate laws. No doubt, there are much more complicated rate laws (where concentration terms are sometimes in the denominator (that is, exponents are negative) or exponents are non integers).
Here is another example:
Experiment
Number[A], M [B], M Intial Rate,
10-5 M/s1 0.100 0.100 4.0 2 0.100 0.200 4.0 3 0.200 0.100 16.0
By comparing 1 and 2, it can be seen that the rate is not dependent on [B]. On the other hand, based on 2 and 3, doubling [A] results in quadrupling the rate. Thus, the reaction is second order with respect to [A] and zero order with respect to [B].
Rate Law: Rate = k [A]2
After determining the rate law, the rate constant k is obtained by dividing each initial rate by its corresponding [A]2.
In this case, experiments 1 through 3 yield 4.0 X 10-3 M-1s-1.
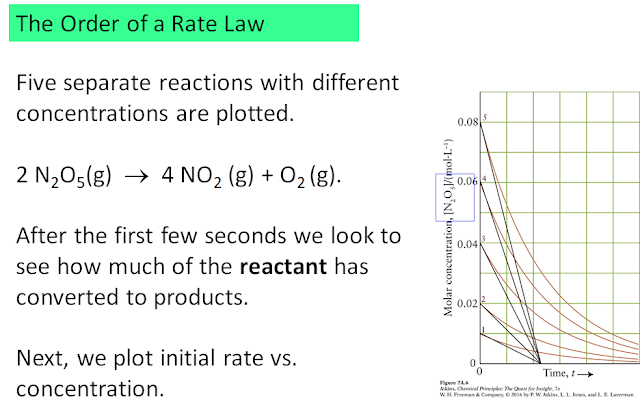
The above consists of five separate experiments, each one starting at a different concentration of the reactant. The rate of disappearance of the reactant N2O5 is monitored over time (red curves), and its instantaneous rate at the start is determined (black lines).
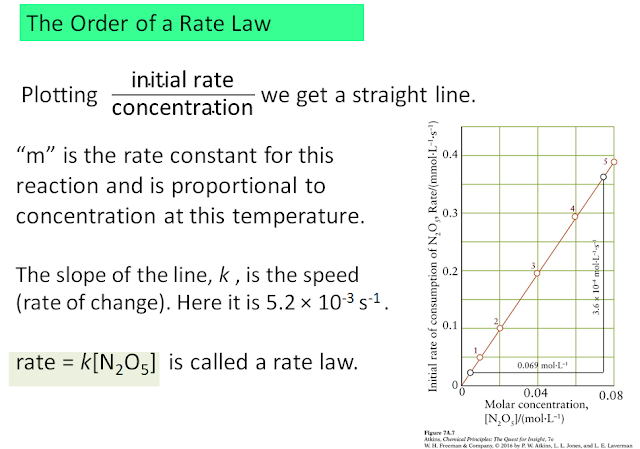
These instantaneous rates at the beginning of the reaction are plotted against the starting concentration:
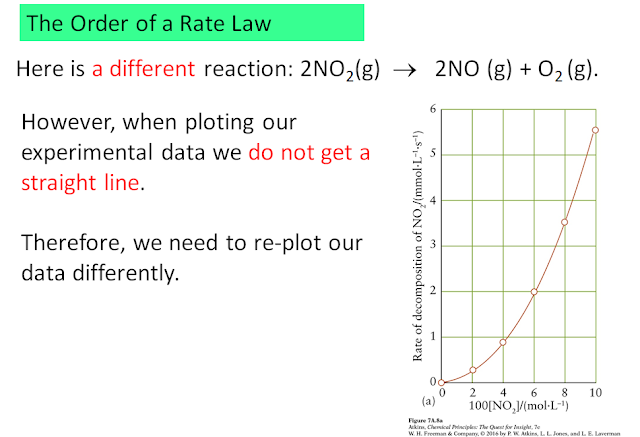
Another example:
The above table for the reaction between hydrogen and iodine also provides as a preview of how sensitive rates of reactions are to temperature.
Example Problems:
Let us continue with more examples for the method of initial rates:
Doubling ammonium doubles the rate, and doubling nitrite also doubles the rate, so this reaction is first order with respect to ammonium, and first order with respect to nitrite. The reaction is second-order overall.
Changing [B] does not change the rate so this reaction is zero-order with respect to [B]. We can then ignore the contribution of changing [B] in experiments 2 and 3, and focus on what [A] does. When [A] is doubled, the rate quadruples so the reaction is second order with respect to [A]. It is second-order overall.
In the above, the reaction is first order with respect to hydrogen (using experiments 1 and 2) and is second order with respect to NO (using experiments 1 and 3). The reaction is third-order overall.
If the experiments are not designed as exact multiples, we can use the logarithm function to extract the order (shown in the above example). The numbers shown above are probably closer (in terms of accuracy) to what you would see in the laboratory.






No comments:
Post a Comment