Chem 1200
Kinetics IV
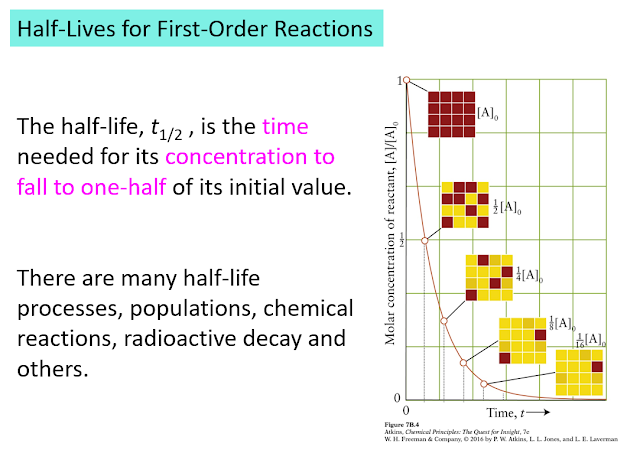
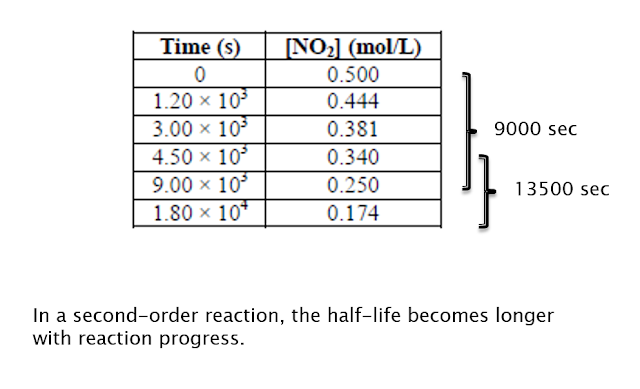
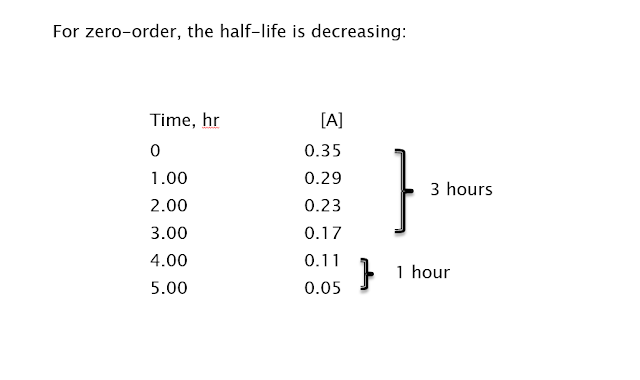
Something Special: Half-lives
We will discuss this in greater detail.But first, here is another example of a first-order reaction, an alkyl bromide (hydrocarbon, that is with C and H's, only single bonds are present, plus a bromide) reacts with water to form an alkene (a hydrocarbon with one C=C, this is formed by elimination of an HBr molecule from the alkyl bromide):
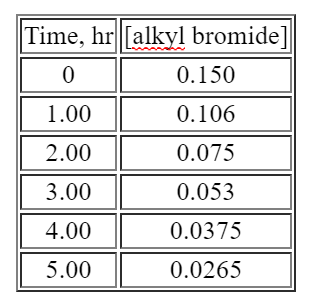
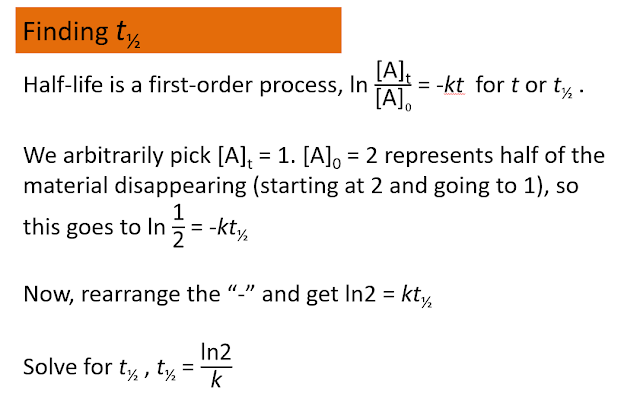
Time, hr [alkyl bromide] 0 0.150 1.00 0.106 2.00 0.075 3.00 0.053 4.00 0.0375 5.00 0.0265 Plot ln[alkyl bromide] versus time and you will see a straight line.Half life - One interesting feature of a first order process involves the time it takes for the starting material to drop to one-half of its initial value. This time is given a special name, half-life. In a first order process, the half-life is independent of the starting concentration, the half-life is a constant:The half-life of a first order process is independent of starting material. It only depends on the rate constant. In fact, using this observation makes it easier to spot a first order process. Look back at the table showing the concentration of alkyl bromide as a function of time. You will see that every two hours the concentration has decreased to half its value.
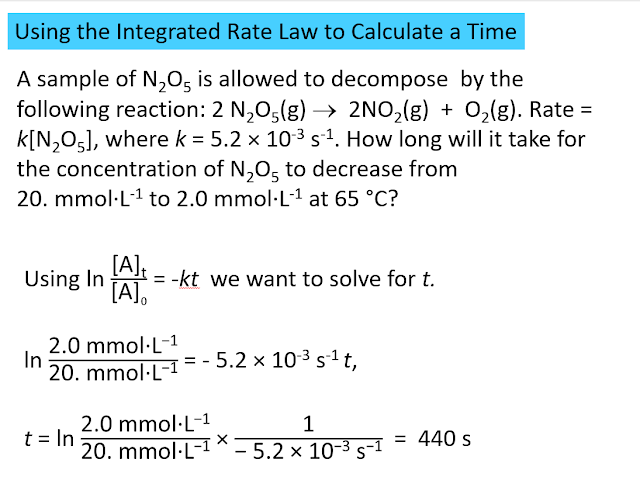
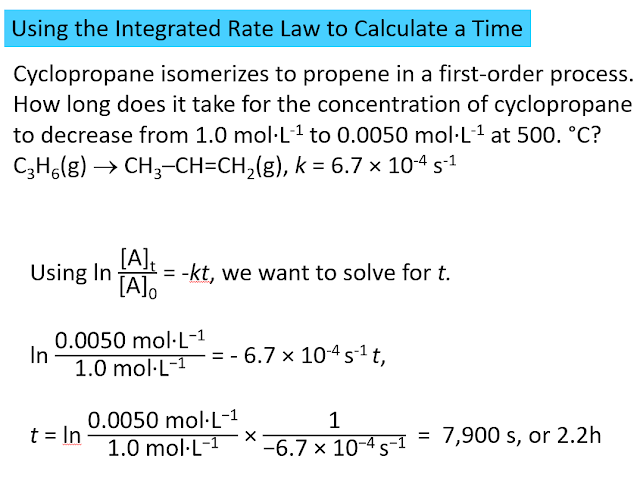
More Sample Problems
Summary The quantitative relationship between the rate of the reaction and the instantaneous concentration of the reactants is expressed in a rate law:Rate of reaction = k [A]m[B]n As written above, the reaction is mth order with respect to reactant A and nth order with respect to reactant B. It is required to determine experimentally the dependence of the rate of the reaction on the concentration of reactants. The experimental determination of the order of the reaction (the powers m and n) usually involves monitoring the rate of the disappearance of one of the reactants (Reactant A) as a function of time while keeping the other reactants (Reactant B) at constant concentration (For example, this can be achieved by using a large excess of the other reactants so that the progress of the reaction hardly changes their concentrations). By making several plots which involve the concentration of reactant A and reaction time, the order of the reaction with respect to [A] (the power m) can be determined. The following relationships are known:
Thus, by simply following the course of a reaction by measuring [A] at specific intervals and trying the 4 different plots and selecting the one which gives a straight line, the order of the reaction with respect to [A] can be obtained.
Order of the
reactionPlot that will yield
a straight lineZero order (m=0) [A] vs. time Half-order (m=1/2) [A]1/2 vs. time First order (m=1) ln [A] vs. time Second order (m=2) [A]-1 vs. time













No comments:
Post a Comment